Radiation therapy
Radiation therapy (also called radiotherapy, X-ray therapy, or irradiation) is the use of ionizing radiation to kill cancer cells and shrink tumors. Radiation therapy can be administered externally via external beam radiotherapy (EBRT) or internally via brachytherapy. The effects of radiation therapy are localized and confined to the region being treated. Although radiation damages both cancer cells and normal cells, most normal cells can recover from the effects of radiation and function properly.
Radiation therapy may be used to treat almost every type of solid tumor, including cancers of the brain, breast, cervix, larynx, lung, pancreas, prostate, skin, stomach, uterus, or soft tissue sarcomas. Radiation is also used to treat leukemia and lymphoma. The radiation dose to each site depends on a number of factors, including the radiosensitivity of each cancer type. Thus, as with every form of treatment, radiation therapy is not without its side effects.
Chemotherapy
Chemotherapy is the treatment of cancer with drugs (“anticancer drugs”) that can destroy cancer cells. In current usage, the term “chemotherapy” usually refers to cytotoxic drugs which affect rapidly dividing cells in general, in contrast with targeted therapy (see below). Chemotherapy drugs interfere with cell division in various possible ways, e.g. with the duplication of DNA or the separation of newly formed chromosomes. Most forms of chemotherapy target all rapidly dividing cells and are not specific to cancer cells, although some degree of specificity may come from the inability of many cancer cells to repair DNA damage, while normal cells generally can. Hence, chemotherapy has the potential to harm healthy tissue, especially those tissues that have a high replacement rate (e.g. intestinal lining). These cells usually repair themselves after chemotherapy.
Because some drugs work better together than alone, two or more drugs are often given at the same time. This is called “combination chemotherapy”; most chemotherapy regimens are given in a combination.[6]
The treatment of some leukemias and lymphomas requires the use of high-dose chemotherapy, and total body irradiation (TBI). This treatment ablates the bone marrow, and hence the body’s ability to recover and repopulate the blood. For this reason, bone marrow, or peripheral blood stem cell harvesting is carried out before the ablative part of the therapy, to enable “rescue” after the treatment has been given. This is known as autologous stem cell transplantation. Alternatively, hematopoietic stem cells may be transplanted from a matched unrelated donor (MUD).
Targeted therapy
Targeted therapy, which first became available in the late 1990s, has had a significant impact on the treatment of some types of cancer and is currently a very active research area. This constitutes the use of agents specific to the deregulated proteins of cancer cells. Small molecule targeted therapy drugs are generally inhibitors of enzymatic domains on mutated, overexpressed or otherwise critical proteins within the cancer cell. Prominent examples are the tyrosine kinase inhibitors imatinib (Gleevec/Glivec) and gefitinib (Iressa).
Monoclonal antibody therapy is another strategy in which the therapeutic agent is an antibody that specifically binds to a protein on the surface of the cancer cells. Examples include the anti-HER2/neu antibody trastuzumab (Herceptin) used in breast cancer, and the anti-CD20 antibody rituximab, used in a variety of B-cell malignancies.
Targeted therapy can also involve small peptides as “homing devices” which can bind to cell surface receptors or affected extracellular matrix surrounding the tumor. Radionuclides which are attached to these peptides (e.g. RGDs) eventually kill the cancer cell if the nuclide decays in the vicinity of the cell. Especially oligo- or multimers of these binding motifs are of great interest since this can lead to enhanced tumor specificity and avidity.
Photodynamic therapy (PDT) is a ternary treatment for cancer involving a photosensitizer, tissue oxygen, and light (often using lasers). PDT can be used as a treatment for basal cell carcinoma (BCC) or lung cancer; PDT can also be useful in removing traces of malignant tissue after surgical removal of large tumors.
Immunotherapy
Cancer immunotherapy refers to a diverse set of therapeutic strategies designed to induce the patient’s own immune system to fight the tumor. Contemporary methods for generating an immune response against tumors include intravesical BCG immunotherapy for superficial bladder cancer, and the use of interferons and other cytokines to induce an immune response in renal cell carcinoma and melanoma patients. Vaccines to generate specific immune responses are the subject of intensive research for a number of tumors, notably malignant melanoma and renal cell carcinoma. Sipuleucel-T is a vaccine-like strategy in late clinical trials for prostate cancer in which dendritic cells from the patient are loaded with prostatic acid phosphatase peptides to induce a specific immune response against prostate-derived cells.
Allogeneic hematopoietic stem cell transplantation (“bone marrow transplantation” from a genetically non-identical donor) can be considered a form of immunotherapy since the donor’s immune cells will often attack the tumor in a phenomenon known as a graft-versus-tumor effect. For this reason, allogeneic HSCT leads to a higher cure rate than autologous transplantation for several cancer types, although the side effects are also more severe.
The cell-based immunotherapy in which the patients own Natural Killer cells(NK) and Cytotoxic T-Lymphocytes(CTL) is used has been in practice in Japan since 1990. NK cells and CTLs primarily kill the cancer cells when they are developed. This treatment is given together with the other modes of treatment such as surgery, radiotherapy, or Chemotherapy and called Autologous Immune Enhancement Therapy(AIET).
Hormonal therapy (oncology)
The growth of some cancers can be inhibited by providing or blocking certain hormones. Common examples of hormone-sensitive tumors include certain types of breast and prostate cancers. Removing or blocking estrogen or testosterone is often an important additional treatment. In certain cancers, the administration of hormone agonists, such as progestogens may be therapeutically beneficial.
Angiogenesis inhibitor
Angiogenesis inhibitors prevent the extensive growth of blood vessels (angiogenesis) that tumors require to survive. Some, such as bevacizumab, have been approved and are in clinical use. One of the main problems with anti-angiogenesis drugs is that many factors stimulate blood vessel growth in cells normal or cancerous. Anti-angiogenesis drugs only target one factor, so the other factors continue to stimulate blood vessel growth. Other problems include the route of administration, maintenance of stability and activity, and targeting the tumor vasculature.
Radiation Therapy
CyberKnife® and Gamma Knife® Radiosurgery – What are they and what are the differences?
The objective of these radiosurgery treatments is to deliver a lethal dose of radiation to only the tumor without affecting surrounding healthy tissue, and there are different ways in which this can be achieved. CyberKnife® and Gamma Knife® are similar in many respects and to help you understand we offer this fact sheet explaining the differences: With regard to the differences between CyberKnife® (CK) and Gamma Knife® (GK) – CyberKnife® uses a former car-building robot to move a LINAC radiotherapy machine around the body in a series of arcs, allowing the multiple beams of radiotherapy to come together at the point of treatment ‘focusing’ the radiotherapy in this way. It does not need a frame to be fitted to the head and can treat anywhere in the body.
CK is relatively new
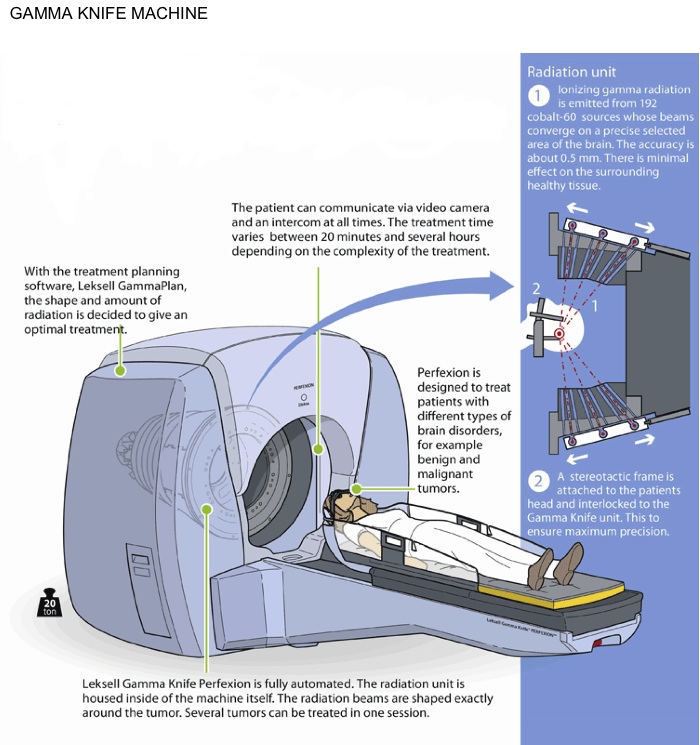
The Gamma Knife® uses a number of fixed radiotherapy sources (192 in the latest ‘Perfexion’) which converge at a single point and the target is placed at this point. It can only treat lesions in the head and neck and requires a frame to be fixed to the patient to achieve the accuracy required for treatment.
Many CK treatments are split into fractions, perhaps 5, and may last longer than GK – partly due to any movement of the patient’ locking out’ the equipment, whereas almost all GK treatments are performed in a single day, lasting only perhaps 30 to 60 minutes.
The CK uses electrically generated radiotherapy through the LINAC (linear accelerator) which has a significant downtime for servicing; the GK sources of radiation are constantly decaying, giving off radioactivity. This leads to a decline in the potency of the GK sources over time and the need to renew the sources every 5 years or so – there is, however, very little machine ‘downtime’.
It should be noted that there are other varieties of LINAC systems which can be used for radiosurgery, including the Varian, this also sometimes requires a frame to be fitted to the head. The CyberKnife® System is a robotic radiosurgery instrument that is revolutionizing the way cancers are treated. It was developed by John Adler, a neurosurgeon from Stanford University in the 1990’s as a way of extending radiosurgery from the head and brain to include the rest of the body. He went on to found Accuray Incorporated, manufacturers of the CyberKnife® System. CyberKnife® is in clinical use throughout the world with over 60,000 patient treatments conducted. Ukraine has three outstanding centers performing this treatment with excellent aftercare.
The Gamma Knife® is a machine designed to deliver radiation in a very precise manner by targeting hundreds of pinpointed beams of radiation directly at the tumor. This is a non-invasive and non-surgical treatment for brain conditions to treat a patient with a single dose over the course of a day rather than in a number of doses over weeks. The early prototype was first used in the late 1960s but was later developed to a model more comparable to today’s machine in the mid-1980s.